
 Favorite (4)
Favorite (4)
The field of Microfluidics and the closely related Organ-on-Chip field are both burgeoning areas for new applications and product development. The number of organizations active in these fields is rapidly increasing, driven by the need for specialized diagnostic devices, reduced costs in medication development, and the ethical imperative to minimize animal testing in pharmaceutical research. Over a longer time horizon, the prospect of personalized medicine and companion diagnostics looms large; both Microfluidics and Organ-on-Chip products are deemed essential for these advancements.
However, these applications are characterized by diversity, presenting suppliers with the challenge of making their products suitable for numerous niche market segments to achieve reasonable production volumes. Additionally, there exists a significant gap between research and industrial fabrication technologies in this domain. Recognizing these challenges, it becomes evident that some form of modular integration, grounded in standardized protocols, is necessary to enable the widespread adoption and successful industrialization of microfluidic instruments across various markets. These standards must delineate the interface between components and Microfluidic/Organ-on-Chip systems to facilitate easy exchangeability (Plug and Play).
This approach not only streamlines component usage across different instruments and applications but also allows for seamless transitions between product development and industrial phases. Crucial to this endeavor are well-defined interface standards and the corresponding agreements on relevant metrology for testing these interfaces.
Driven by the burgeoning interest in standardization within microfluidics and Organ-on-Chip, a consortium of experts convened last November for a pivotal workshop. Drawing participants from 10 countries, totaling 45 attendees, the workshop featured presentations by industry stalwarts in metrology, regulation, microfluidics, Organ-on-Chip product development, and semiconductor technologies. These presentations encompassed ongoing standardization and metrology initiatives, each followed by a round table discussion honing in on specific topics. Through these discussions, it became evident that a multitude of metrology and reliability issues necessitate collaborative efforts across organizations, countries, and continents. Several key initiatives emerged from the dialogue:
Furthermore, noteworthy efforts in the USA are spearheaded by the FDA and NIST.
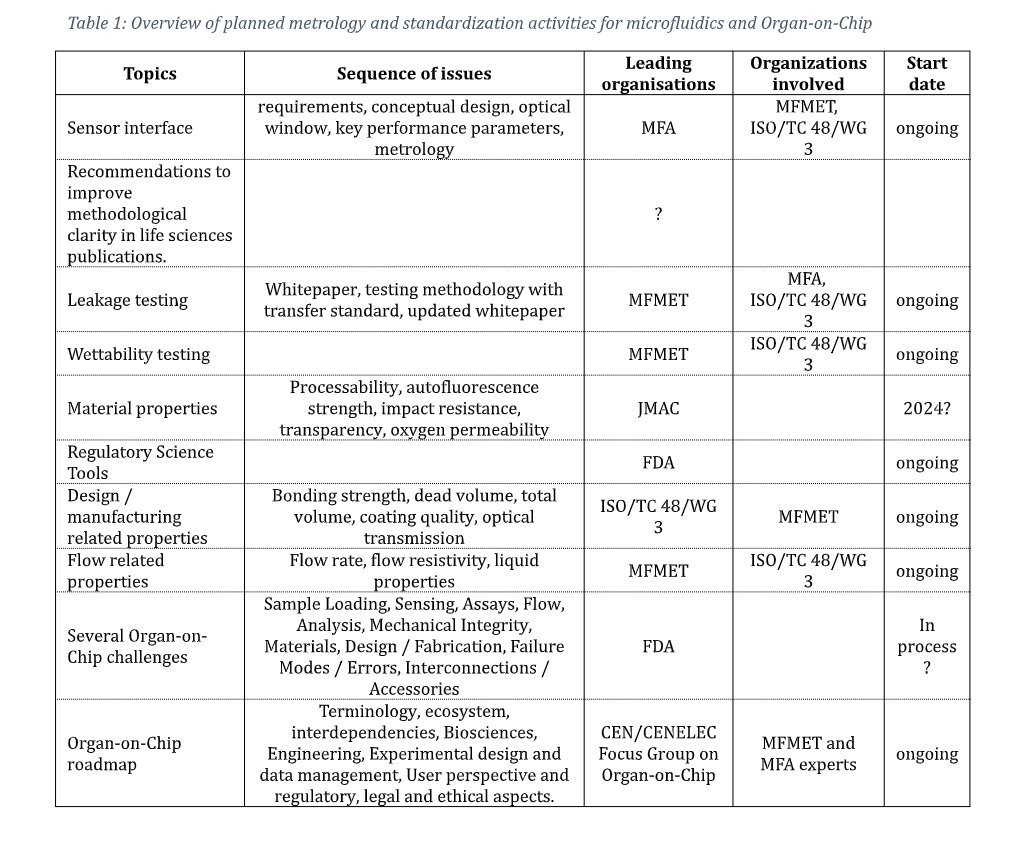
For a comprehensive overview of ongoing and planned metrology and standardization activities in microfluidics and Organ-on-Chip, refer to the table below.
Typically, a microfluidic setup consists of several microfluidic components connected by tubing:
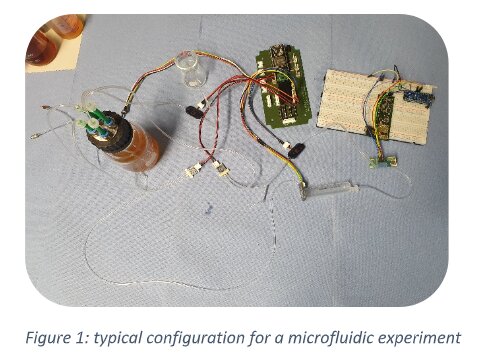
This way, the researchers have freedom to select the best components and optimal design for their experimental setup. A disadvantage is the diversity of connection systems. Also, connection systems in use by the community are developed for other industrial areas and are suboptimal for microfluidic and Organ-on Chip applications.
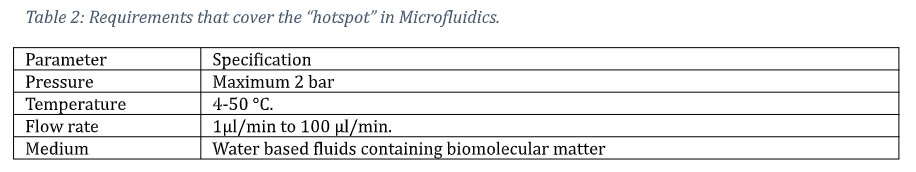
A group of over 30 companies, all major suppliers of micropumps, flow sensors, microfluidic valves, chips etc., supported by the Microfluidics Association and the MFMET project, came to an agreement about a generic microfluidic connection system. This system is designed in such a way that it adheres to the requirements for the “hotspot” in microfluidics, i.e., the area where most of the Microfluidic an Organ-on-Chip are designed for.
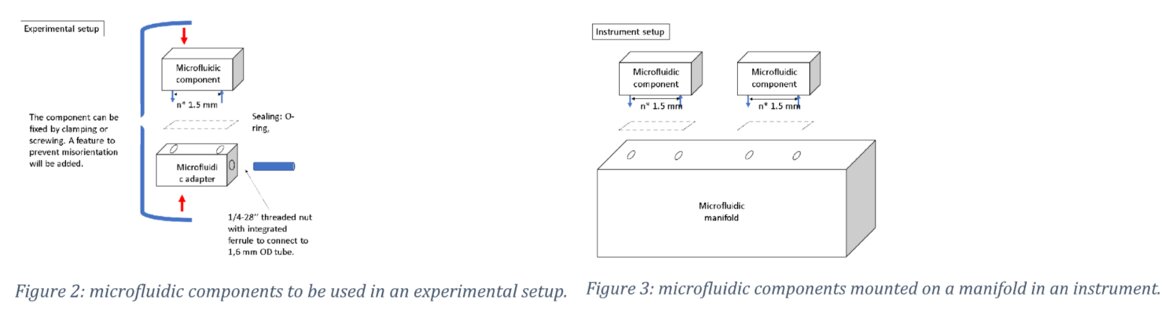
To enable seamless transfer between the experimental phase and industrial set up a top-down approach of mounting the components was chosen, offering a path towards further integration where the components are fixed on a manifold, without tubing (see below).
For both approaches the same components can be used. This approach limits the difference between the experimental conditions and the conditions in the final instrument version, while the same microfluidic components can be used in both cases.
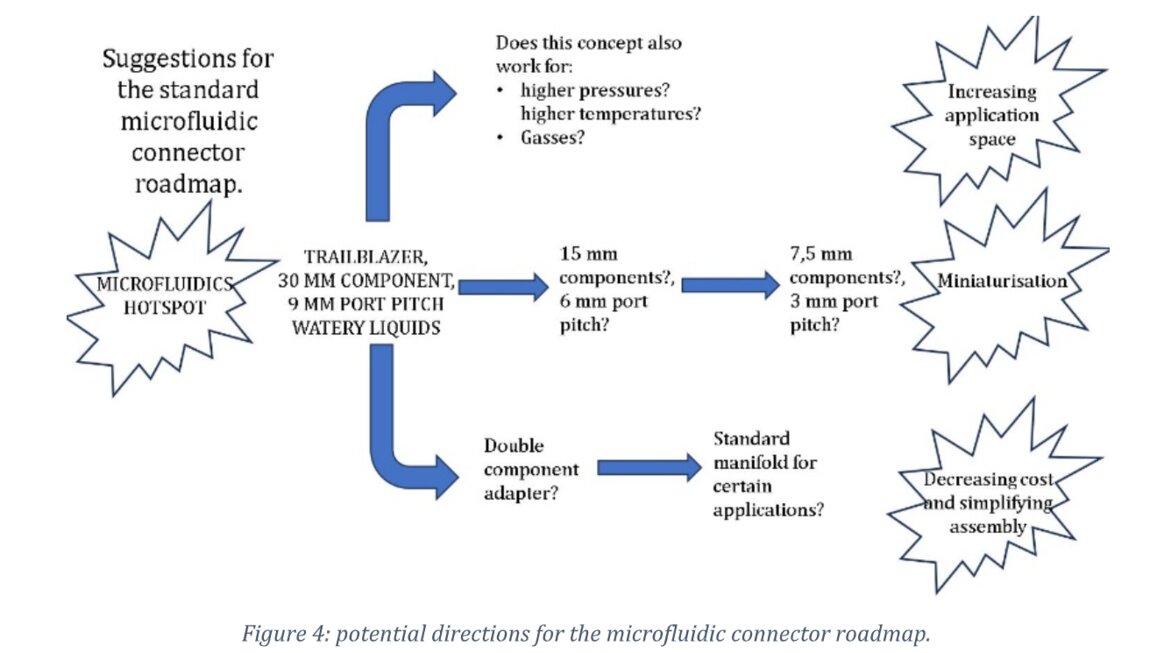
It must be realized that there are many potential applications outside of the identified hotspot and therefor it was proposed to develop a roadmap for future applications and product generations. Elements of that this roadmap should cover:
A very large thank you to all the industry experts who patiently filled in surveys and participated in many online and life discussions. This work was not possible with the cooperation of members of the Microfluidics Association and financial support from the 20NRM02 MFMET project that funded by the EMPIR programme co-financed by the Participating States and from the European Union’s Horizon 2020 research and innovation programme.